|




| |
 |
LCM:
The Law of Conservation of Mass
states that the mass of the reactants is equal to the mass of the products
|
 |
LCC:
The Law of Constant Composition
states that the ratio of any compound with respect to the elements that make
up the compound is fixed (constant)
|
 |
LMP:
The Law of Multiple Proportions
states that compounds form according to simple, whole number ratios
|
 |
LCE:
The Law of Conservation of Energy
|
 |
Atomic
Weight =
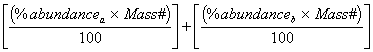
|

The
Mole:

 |
Percentage
Yield: [actual amount / theoretical amount]
X 100%
|



|
